Structural Insights into How Ions in Basic Solutions Transform
Identified a new intermediate phase that forms during the crystallization of hydrated aluminum-containing salts
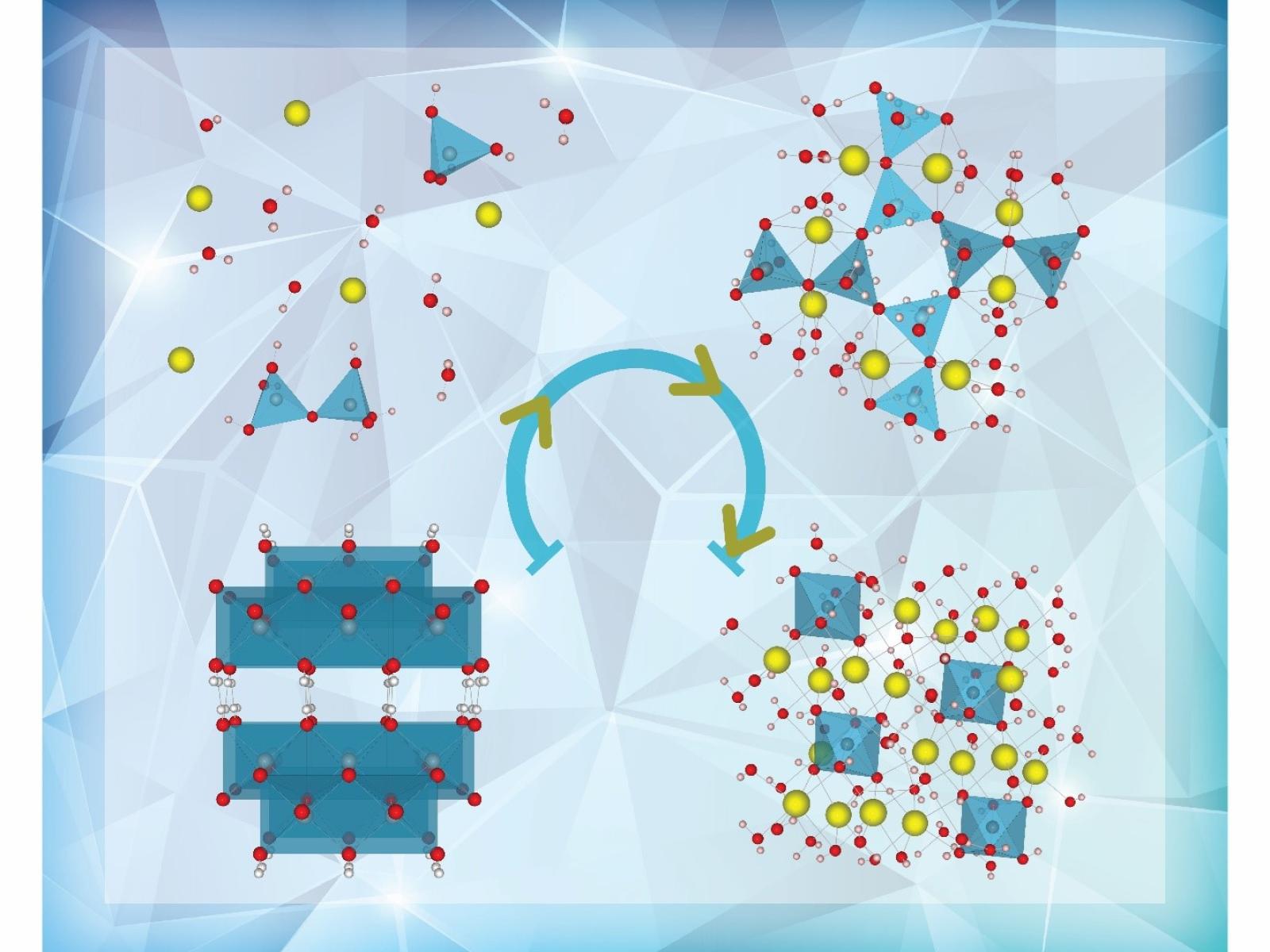
As hydrated aluminum-containing salts crystallize from solution, an intermediate highly linked tetrahedral aluminate phase forms. The lower left represents the starting mineral and the lower right the final crystalline salt.
(Illustration by Jamie Gority | Pacific Northwest National Laboratory)
The Science
Understanding the behavior of hydrated aluminum-containing salts in very basic (alkaline) solutions is important for industrial processes, including aluminum (Al) refining and nuclear waste processing. However, the molecular-level details of crystallization and dissolution remain unclear. Melting together hydrated sodium hydroxide and an aluminum oxyhydroxide mineral called boehmite allowed researchers to probe the structure of hydrated aluminate salts as they transform. Using nuclear magnetic resonance spectroscopy, X-ray diffraction, and Raman spectroscopy, the research team identified a new highly linked intermediate. This intermediate phase has a different aluminum structure than either the starting mineral or final salt, acting as a transition between the two.
The Impact
Hydrated aluminum-containing salts are found in legacy radioactive tank waste, including those at the Hanford Site. Understanding how these salts behave at a molecular level will help workers better predict waste characteristics important to processing. This work provides insights into how dissolved aluminum in the form of tetrahedral aluminate converts into octahedral solids under very basic conditions, including identifying new temporarily stable intermediates. These intermediates help facilitate the transformation of aluminum from one coordination environment to another, which may have similarities to how aluminum minerals form and dissolve.
Summary
In highly basic solutions, aluminum containing minerals can transform into hydrated salts. This process, while important for industrial and waste remediation applications, remains poorly understood at the molecular level. New research explored the transformation of an aluminum oxyhydroxide mineral into a hydrated aluminate salt under highly basic conditions. Scientists heated solid boehmite and sodium hydroxide monohydrate. They observed the formation of a previously unseen intermediate phase. This phase contained temporarily stable aluminum compounds, with the bonding geometry around the aluminum taking on a structure with a high degree of condensation. Experimental probes showed that the intermediate compound has an amorphous, chain-like structure. The initial discoveries in this work provide guidance for further studies to interrogate the structure of the identified intermediates with ultrafast spectroscopy.
PNNL Contact
Trent R. Graham, Pacific Northwest National Laboratory, trenton.graham@pnnl.gov
Carolyn Pearce, Pacific Northwest National Laboratory, carolyn.pearce@pnnl.gov
Funding
This research was supported by IDREAM (Interfacial Dynamics in Radioactive Environments and Materials), an Energy Frontier Research Center funded by the Department of Energy, Office of Science, Basic Energy Sciences program. This research used resources at EMSL, the Environmental Molecular Sciences Laboratory, a Department of Energy Office of Science user facility at Pacific Northwest National Laboratory.
Published: December 2, 2022
Graham, T. R., J. Z. Hu, N. R. Jaegers, X. Zhang, C. I. Pearce, K. M. Rosso. 2022. “An Amorphous Sodium Aluminate Hydrate Phase Mediates Aluminum Coordination Changes in Highly Alkaline Sodium Hydroxide Solutions,” Inorganic Chemistry Frontiers, Advance Article. [DOI: 10.1039/D2QI01642G]