In Vitro Dosimetry
In Vitro Dosimetry
Particle Dosimetry for In Vitro Particle Toxicology Systems
ISDD Development Funded
by NIEHS (ES016212)
The dose-response paradigm for the fields of pharmacology and toxicology are predicated on the principle that response is proportional to the concentration of the effector molecule at the site of action. The use of target tissue dose, rather than less specific measures of “dose” such as exposure or administered dose, improves correlations between dose and response for drugs, chemicals, and inhaled gases and particles.
The dynamics of particles in liquids are well studied and mathematical approaches for describing both diffusion and gravitational settling have been developed.
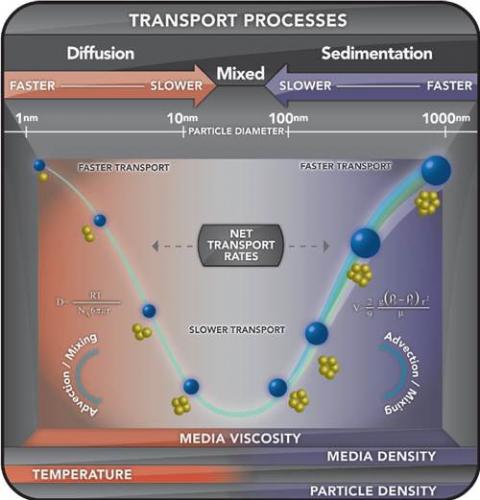
These approaches were used to develop a method for describing the particokinetics–the combined influence of diffusion and gravitational settling—on particle transport to cells in vitro. The In Vitro Sedimentation, Diffusion and Dosimetry (ISDD) model is a computational model of particokinetics (sedimentation, diffusion) and dosimetry for noninteracting spherical particles and their agglomerates in cell culture systems common to toxicology. The model is developed from first principles–Stokes sedimentation and Stokes-Einstein diffusion–and verified against experimentally measured rates of nano- and microparticle transport across several particles sizes, and densities as well as agglomerates. ISDD is an in vitro counterpart to the Multipath Particle Deposition Model (MPPD) for inhaled particles and can be used with MPPD to related in vitro doses to cellular doses of inhaled particles.
Uses
ISDD is used to estimate the amount of particles reaching cells residing at the bottom of a conventional cell culture system during a defined exposure period. The model reports the fraction of particles, the surface area, mass and number of particles reaching cells as well as the Area Under the Time-Delivered Dose Curve (AUC).
Where measurement of nanoparticle cellular dose is not possible/practical, ISDD can be used to compare particle doses across particle types within a system, and compare particle doses between systems with different characteristics (media height, viscosity, orientation).
Applied in conjunction with in vivo dosimetry models such as the Multipath Particle Deposition Model (MPPD), ISDD can be used as the initial step in translating cellular doses of nanoparticles between in vitro and in vivo systems (Teeguarden et al., 2014). This application area can grow as models of systemic particokinetics emerge.
Example Applications
Smith, J. N., D. G. Thomas, H. Jolley, V. K. Kodali, M. H. Littke, P. Munusamy, D. R. Baer, M. J. Gaffrey, B. D. Thrall and J. G. Teeguarden (2018). "All that is silver is not toxic: silver ion and particle kinetics reveals the role of silver ion aging and dosimetry on the toxicity of silver nanoparticles." Part Fibre Toxicol 15(1): 47.
Peuschel, H., T. Ruckelshausen, C. Cavelius, and A. Kraegeloh, Quantification of Internalized Silica Nanoparticles via STED Microscopy. Biomed Res Int, 2015. 2015: p. 961208.
Cohen, J.M., J.G. Teeguarden, and P. Demokritou, An integrated approach for the in vitro dosimetry of engineered nanomaterials. Part Fibre Toxicol, 2014. 11: p. 20.
Herzog, F., K. Loza, S. Balog, M.J. Clift, M. Epple, P. Gehr, A. Petri-Fink, and B. Rothen-Rutishauser, Mimicking exposures to acute and lifetime concentrations of inhaled silver nanoparticles by two different in vitro approaches. Beilstein J Nanotechnol, 2014. 5: p. 1357-70.
Mahnama, A., G. Ghorbaniasl, S.M. Allaei, and A. Nourbakhsh, Semi-analytical solution for the in-vitro sedimentation, diffusion and dosimetry model: surveying the impact of the Peclet number. Colloids Surf B Biointerfaces, 2014. 122: p. 324-31.
Larson, J.K., M.J. Carvan, 3rd, J.G. Teeguarden, G. Watanabe, K. Taya, E. Krystofiak, and R.J. Hutz, Low-dose gold nanoparticles exert subtle endocrine-modulating effects on the ovarian steroidogenic pathway ex vivo independent of oxidative stress. Nanotoxicology, 2014. 8(8): p. 856-66.
Sharma, G., V. Kodali, M. Gaffrey, W. Wang, K.R. Minard, N.J. Karin, J.G. Teeguarden, and B.D. Thrall, Iron oxide nanoparticle agglomeration influences dose rates and modulates oxidative stress-mediated dose–response profiles in vitro. Nanotoxicology, 2014. 8(6): p. 663-675.
Teeguarden, J.G., V.B. Mikheev, K.R. Minard, W.C. Forsythe, W. Wang, G. Sharma, N. Karin, S.C. Tilton, K.M. Waters, B. Asgharian, et al., Comparative iron oxide nanoparticle cellular dosimetry and response in mice by the inhalation and liquid cell culture exposure routes. Part Fibre Toxicol, 2014. 11(1): p. 46.
Ahmad Khanbeigi, R., A. Kumar, F. Sadouki, C. Lorenz, B. Forbes, L.A. Dailey, and H. Collins, The delivered dose: Applying particokinetics to in vitro investigations of nanoparticle internalization by macrophages. J Control Release, 2012. 162(2): p. 259-66.