The Primary Gas Phase Hydration Shell of Hydroxide
A combined experimental and computational approach showed that the hydration shell of hydroxide has a four-coordinate binding
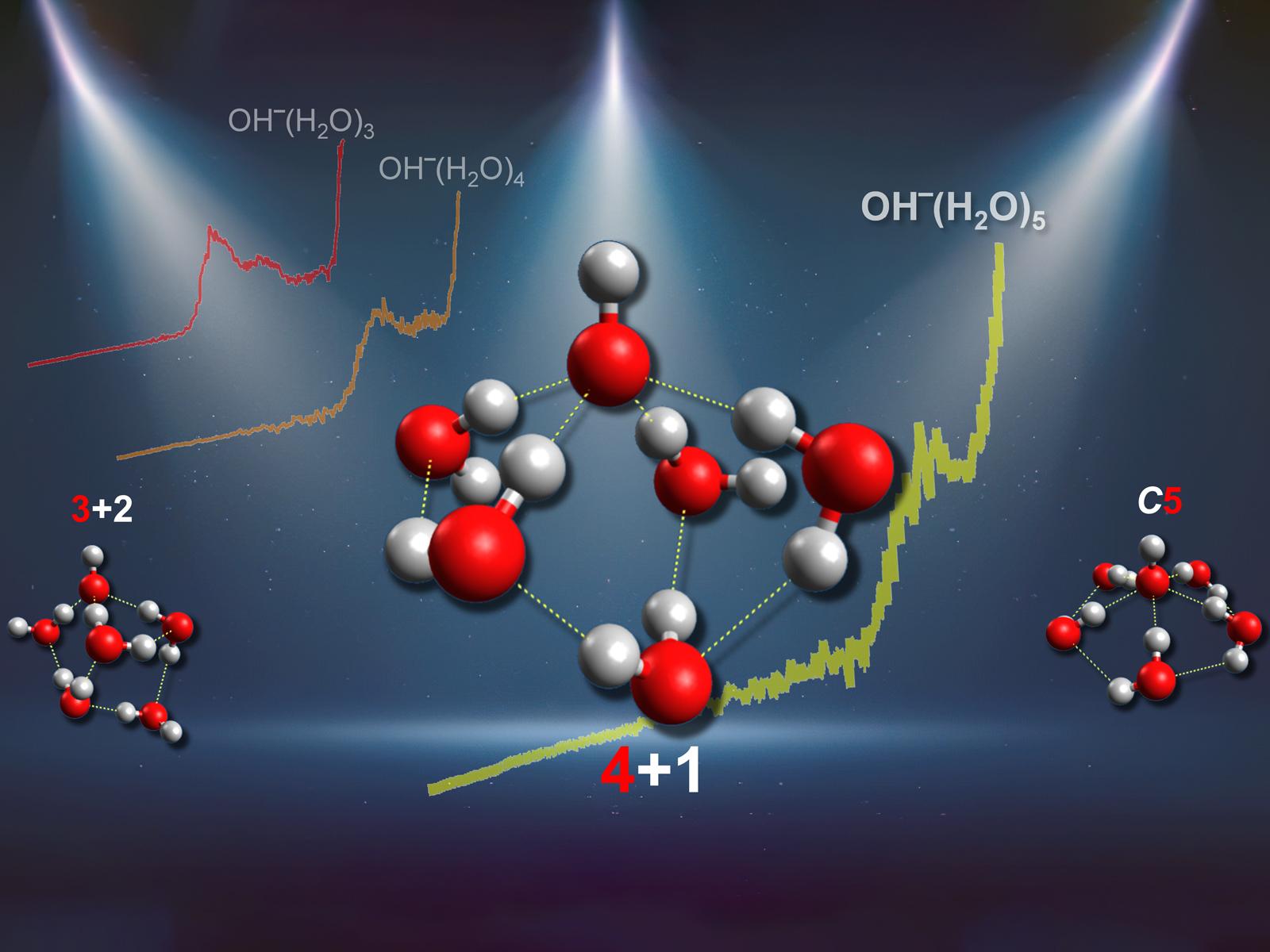
Both spectra and simulations support a four-coordinate binding structure around hydroxide clusters.
(Image by Wenjin Cao | Pacific Northwest National Laboratory)
The Science
Hydroxide is an incredibly common molecular anion, yet its fundamental behavior in water remains unclear. Specifically, the number of water molecules in hydroxide’s primary hydration shell has been the subject of debate. Previous research presented experimental infrared spectroscopy data suggesting three waters, but theoretical studies suggested four. This work provides direct evidence for the presence of a fourth water molecule by combining photoelectron spectroscopy and theoretical computations. Performing experiments at extremely low temperatures allowed researchers to obtain the resolution necessary to identify the electron binding energies, while carefully benchmarked calculations provided the theoretical data.
The Impact
Understanding the behavior of hydroxide surrounded by water is essential for accurately describing many aqueous chemical processes. The fundamental question of how water interacts with hydroxide can affect studies, both experimental and computational, of other systems that involve water and hydroxide. This is particularly relevant for reactions that include acid-base chemistry or take place in complex ionic systems. The results of this work will also help researchers more accurately study proton and hydroxide diffusion dynamics.
Summary
Hydroxide is one of the most important ions in aqueous chemistry. It controls acid-base chemistry, affects the speciation and solvation dynamics of ionic solutions, and participates in additional critical processes in aqueous systems. A key property associated with the behavior of hydroxide in aqueous solutions is the structural motif of its primary hydration shell. However, there have been long-term debates on the number of water molecules in the shell. The only previous experimental research supported a shell with three waters, contradicting the results of most theoretical studies that indicated the shell contained four water molecules.
The previous experimental study used infrared vibrational spectroscopy to determine whether water molecules were directly bonded to hydroxide or reside in the second hydration shell. The study looked for the appearance of water-water bands, indicating water in the second shell. However, the possible existence of these inter-water hydrogen bonds in larger OH–(H2O)n clusters limits their structural assignment via traditional infrared spectroscopy methods. A joint experimental–theoretical approach, based on never-before-reported photoelectron spectra and high-level ab initio calculations for hydrated hydroxide clusters, provides direct and unambiguous evidence for a four-coordinated gas phase hydroxide primary solvation shell. These findings agree with the structural motifs suggested in previous studies using experimental neutron diffraction data and molecular simulations of aqueous hydroxide solutions. The combined results suggest similarities in water coordination structures around hydroxide in clusters and solution phase environments.
PNNL Contact
Xue-Bin Wang, Pacific Northwest National Laboratory, xuebin.wang@pnnl.gov
Sotiris Xantheas, Pacific Northwest National Laboratory, sotiris.xantheas@pnnl.gov
Funding
This work was supported by Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences, Condensed Phase and Interfacial Molecular Science program, FWP 16248, Molecular Theory and Modeling, FWP 16249.
Published: March 24, 2023
Cao, W., Wen, H., Xantheas, S. S., Wang, X.-B. 2023. “The Primary Gas Phase Hydration Shell of Hydroxide.” Sci. Adv. 9, eadf4309. [DOI: 10.1126/sciadv.adf4309]