Predicting the Thermodynamics and Kinetics of Catalytic Hydrogenation
A new approach identifies activity descriptors for investigating catalysts to store energy via hydrogenation
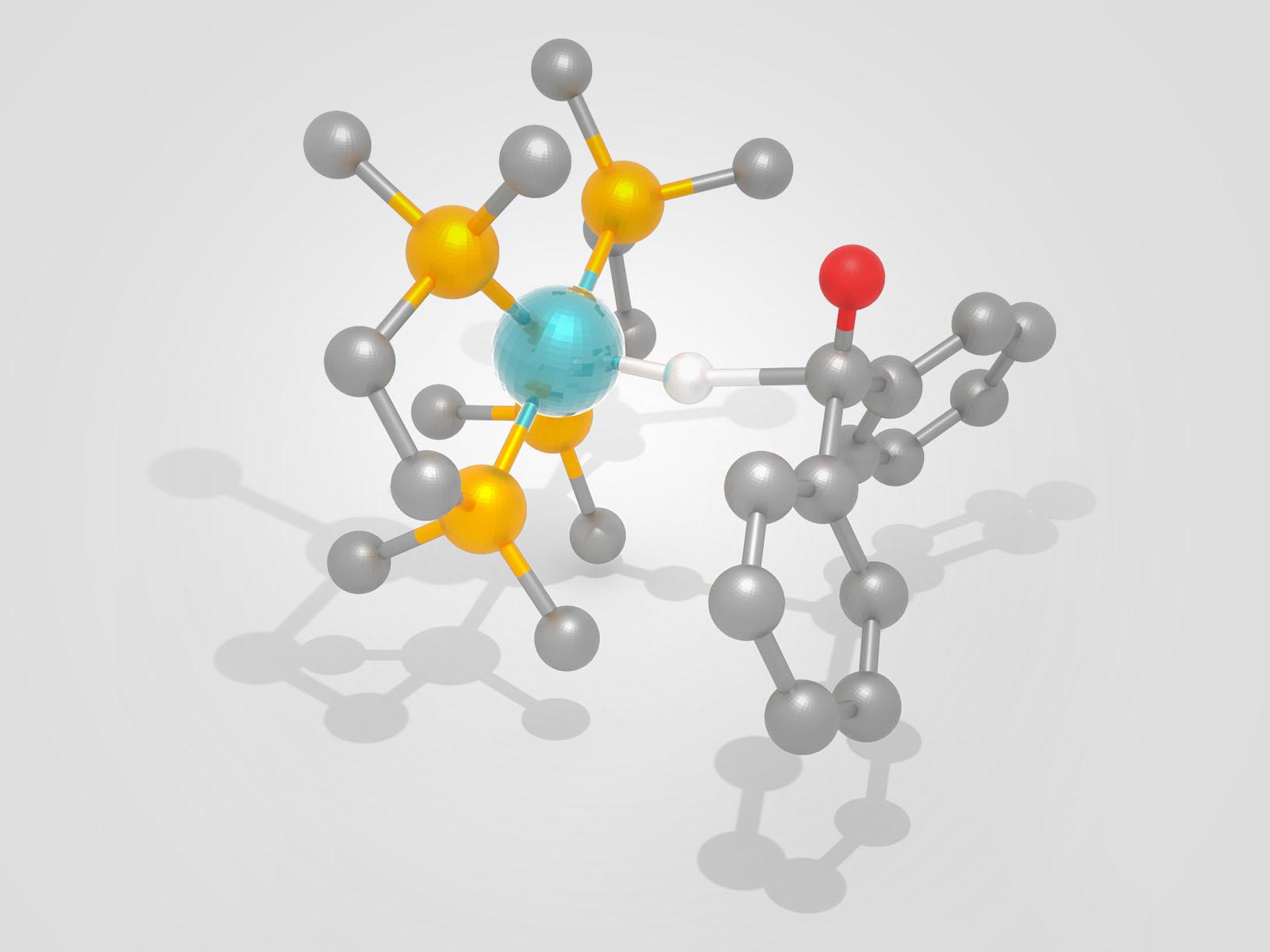
A new study identifies catalytic activity descriptors that can accelerate development of novel catalysts that utilize hydrogen more efficiently. Shown here is a representation of hydride transfer between a catalyst (L) and a substrate (R), a key step in hydrogenating organic molecules.
(Image by Eric Wiedner & Shannon Colson | Pacific Northwest National Laboratory)
The Science
Significant effort has been devoted to understanding how hydrogen can be safely stored in organic hydrogen carriers (OHCs) such as ketones. However, catalysts are needed that can efficiently hydrogenate these carriers. Activity descriptors are a powerful tool for investigating the ability of specific catalysts to hydrogenate OHCs. This study develops two descriptors—hydricity and hydride self-exchange rate—for ketone hydrogenation. Hydricity is a thermodynamic term for how strongly a molecule can bind hydride (H−), and hydride self-exchange rate is a kinetic term for how fast a molecule can transfer H−. Researchers found that by using both descriptors, the activity of a rhodium catalyst could be accurately predicted.
The Impact
Hydrogen is a key industrial feedstock that is primarily derived from fossil carbon, and more sustainable generation and utilization of hydrogen will be essential for the transition to a net-zero economy. Catalysts that can effectively and efficiently transfer hydrogen for storage in OHCs are a key step. This study provides critical information by identifying activity descriptors that can accelerate the development of new catalysts that utilize hydrogen more efficiently with less wasted energy.
Summary
Hydricity is an effective descriptor for understanding and predicting the thermodynamics of the transfer of hydride (H−) from one molecule to another. However, the broad use of hydricity as a descriptor for catalytic reactions has been limited by a lack of values for organic substrates and its insufficiency for predicting the kinetics of a new catalyst. This study investigated the use of thermodynamic and kinetic descriptors for hydride transfer between a rhodium catalyst and a series of ketone substrates. Researchers expanded the range of suitable organic substrates by determining hydricity values for 46 ketone/alkoxide pairs using a combination of experimental measurements and electronic structure calculations. A second descriptor, the rate at which a molecule can exchange a H– with itself, was measured for the rhodium catalyst and the ketone substrates. By combining these descriptors in a model based on the Marcus theory for electron transfer, hydricity and the hydride self-exchange rate can accurately predict the rate of hydride transfer during the catalytic hydrogenation of ketones. This work greatly improves the toolbox for designing catalysts that can efficiently hydrogenate organic molecules.
PNNL Contact
Eric Wiedner, Pacific Northwest National Laboratory, Eric.Wiedner@pnnl.gov
Funding
This work was funded by the Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, Catalysis Science program, FWP 47319.
Published: April 5, 2024
Chirila, A., Y. Hu, J. Linehan, D. Dixon, and E. Wiedner. 2024. “Thermodynamic and Kinetic Activity Descriptors for the Catalytic Hydrogenation of Ketones,” Journal of the American Chemical Society, [DOI: 10.1021/jacs.3c13876]