Lithiation-Assisted Epitaxy for Materials Discovery
Understanding how lithium moves to create new energy storage materials and devices
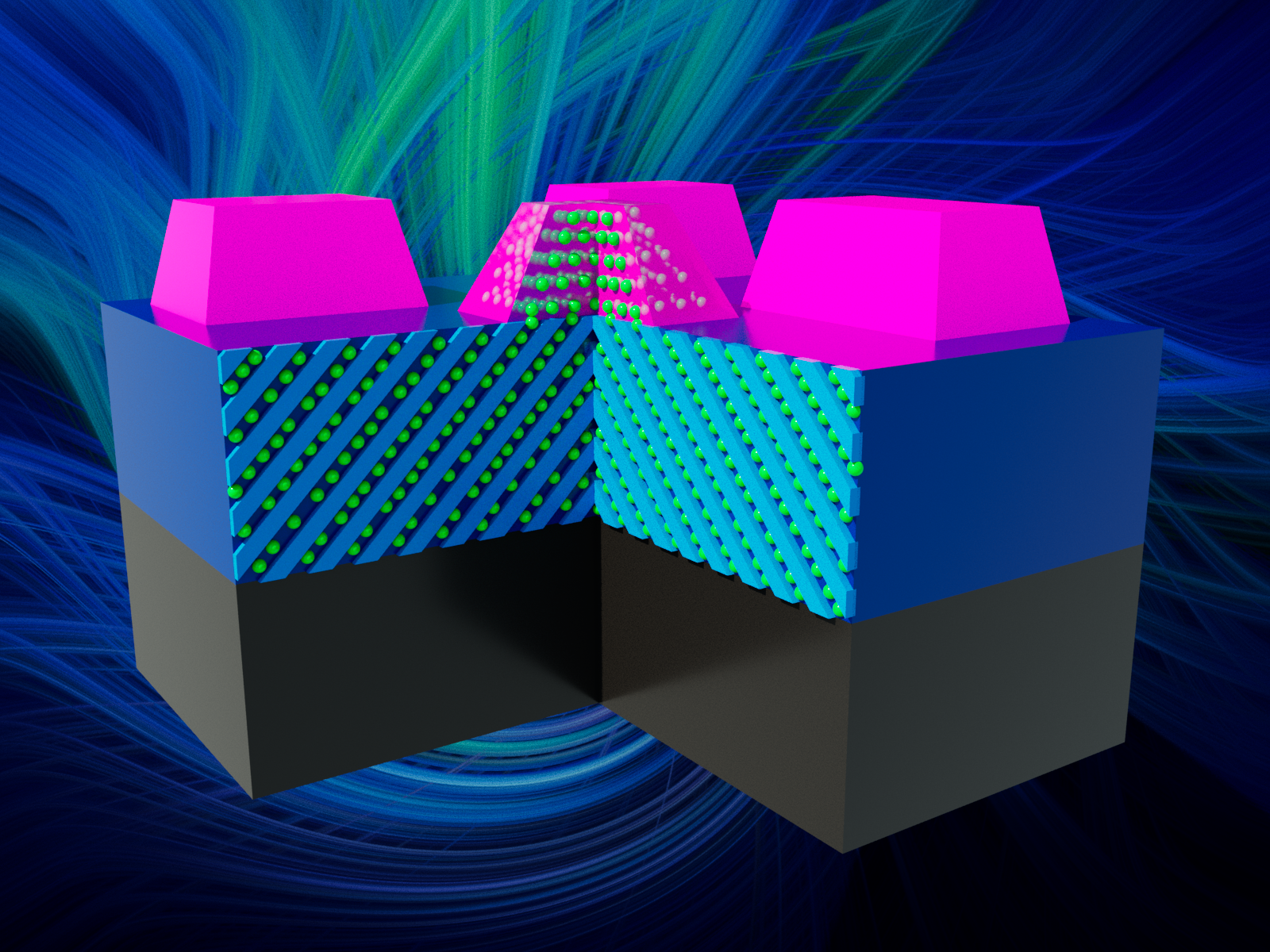
Lithium ions can move into growing materials, creating new and more complex structures.
(Illustration by Cortland Johnson | Pacific Northwest National Laboratory)
Fully solid lithium-based energy storage devices are promising systems for next-generation electric vehicles. However, they require discovering and understanding new materials that can host and transport lithium ions.
Oxide materials, composed of oxygen and one or more metals, are stable, easy to handle, and relatively low cost. These properties make them attractive for applications within solid-state energy storage devices. But these devices have complicated structures, with layers of different materials.
A major research focus has been exploring what happens at the places where these different materials touch, known as interfaces. As energy storage devices rely on the movement of lithium ions, understanding how ions move from one material to another is key to developing successful devices.
An international research team led by Pacific Northwest National Laboratory (PNNL) scientists developed a new approach to study how lithium ions move through oxide materials in a paper published in Nano Letters. Through epitaxial growth, which forms new crystalline layers with a clear orientation atop a crystalline seed layer, the team created structures with multiple stacked oxides.
While many scientists grow epitaxial oxides to create precisely defined interfaces, the PNNL researchers’ approach differs in how it takes advantage of ion movement through materials. The team found that lithium ions moved across the interface to the other side to form new single crystalline materials.
“We used a two-step method to facilitate ion movement,” said Le Wang, a PNNL materials scientist and lead author of the paper. “We first deposited a lithium-based layer, followed by a second oxide without lithium.”
The researchers used lithium cobalt oxide, a common cathode material in energy storage devices, as the base lithium-containing layer. They then performed two sets of experiments using different simple oxides, tungsten oxide (WO3) and titanium dioxide (TiO2). They deposited the oxide on top of the lithium-containing base to produce a layered oxide. Depending on the starting material, final top layer was either lithium tungsten oxide (Li2WO4) or lithium titanium oxide (Li4Ti5O12).
Understanding how lithium moves
While identifying the overall composition of the material was relatively straightforward, understanding how the structure formed was not. There are many potential paths that could produce the lithium-containing oxides.
One option has the tungsten or titanium oxide depositing as a pure layer on the surface. The lithium ions would stay in its original layer forming a clearly defined interface.
Another option has the lithium at the topmost surface layer reacting with the incoming material. This creates new chemical bonds and an integrated layer of a new oxide. However, the amount of lithium at the surface is limited.
The research team found that the lithium adds to the oxide to a much higher degree. The lithium from the entire lower layer can move through the material. It can then react with the arriving tungsten or titanium oxide on the surface. This creates a new oxide with lithium fully integrated into the material. The hallmark of this pathway is a change in the amount of lithium in the bottom layer in addition to a new crystalline oxide that has lithium incorporated.
While this work primarily explores tungsten and titanium oxides, the researchers showed that the same method can be extended to other oxides, including molybdenum and iron oxides. The ability to add lithium to a range of different oxide materials enables the discovery and study of complex oxide-based energy materials and structures.
“In addition to developing new materials, we can now precisely make model interfaces and multilayers to study how ions move in energy storage devices,” said PNNL materials scientist Yingge Du. “Interfaces in practical devices are complex. Examining these epitaxial interfaces can help us get an atomic-level understanding of these processes.”
This research was supported by the Department of Energy, Basic Energy Sciences program’s, Materials Sciences and Engineering Division. In addition to Wang and Du, the PNNL team included Zhenzhong Yang, Widitha Samarakoon, Yadong Zhou, Mark Bowden, Jinhui Tao, Zihua Zhu, Nabajit Lahiri, Timothy Droubay, and Scott Chambers. Collaborating institutions were Argonne National Laboratory, Binghamton University, Oregon State University, and the National University of Singapore.
Published: August 2, 2022