Liquid Porosity May Be Key to More Efficient Battery
PNNL study shows promise for electrolyte design
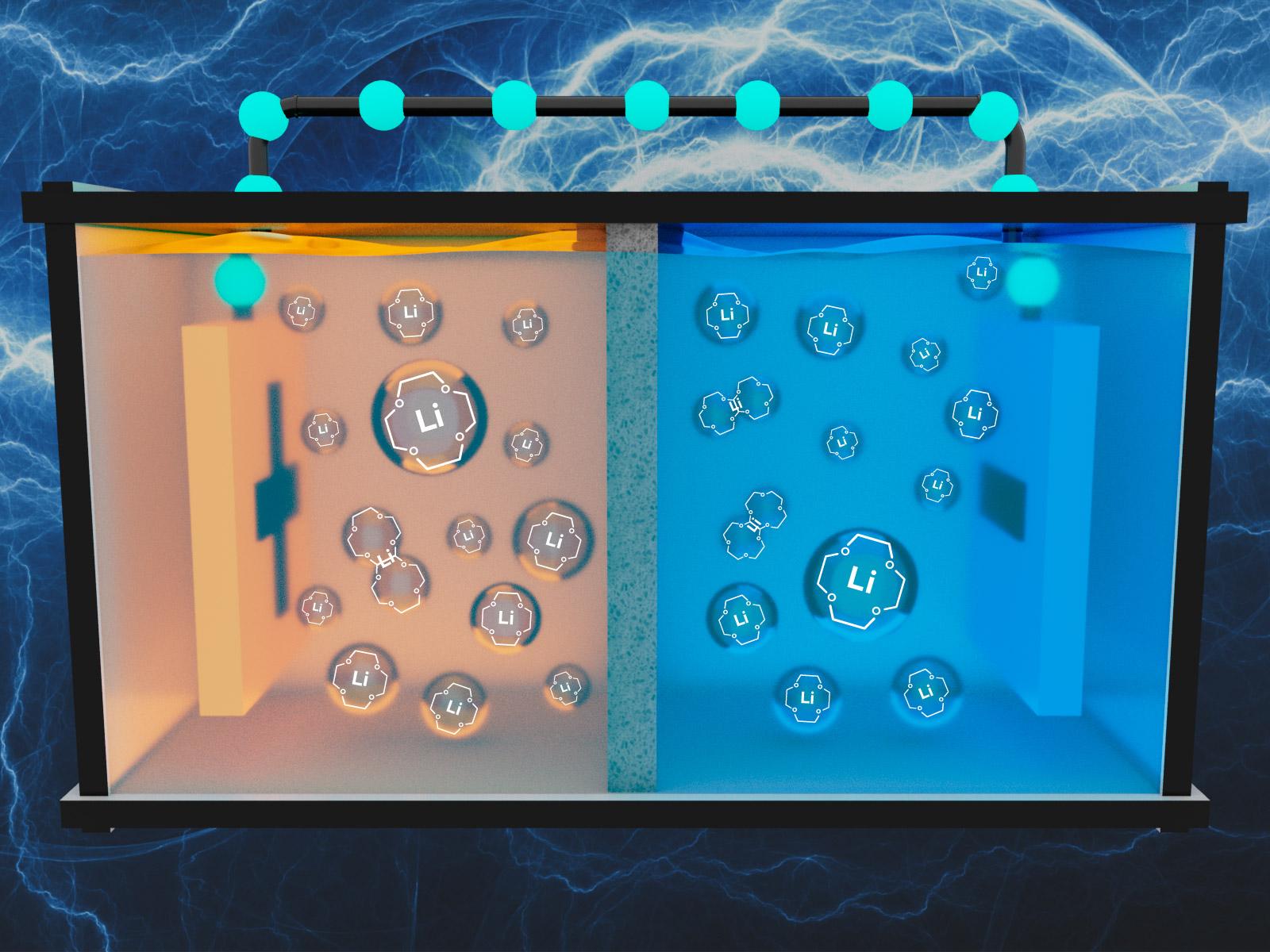
PNNL researchers have designed liquid-based porous electrolytes that could transport lithium ions more easily between electrodes, improving battery efficiency.
(Illustration by Cortland Johnson | Pacific Northwest National Laboratory)
Electrolytes are the blood of a battery. Anything that makes those electrolytes move more easily will likely produce a battery that performs at top efficiency.
That was the hypothesis and motivation for Pacific Northwest National Laboratory (PNNL) researchers who designed a liquid-based porous electrolyte that would transport lithium ions more easily between electrodes. Successful application of the theory would have positive implications for rechargeable lithium batteries, which are widely used as power sources for portable devices and electric vehicles.
“The job of the electrolyte in this type of battery is basically to transport lithium from one side to the other side,” said PNNL physicist Vijay Murugesan, a staff scientist and Materials Sciences group leader in the Physical Sciences Division and one of the authors of the study published in an American Chemical Society journal. The study is titled “Porous Liquids as Electrolyte: A Case Study of Li+ and Mg2+ Ion Transport in Crown Ether-Based Type-II Porous Liquids.”
Microscopic pores are central to the PNNL scientists’ theory. Their study shows that precisely designed pores within a liquid electrolyte can more efficiently move lithium ions. The researchers also showed energy efficiency improvements with magnesium ions because of the potential to replace scarce lithium resources in future energy storage development.
The researchers believe their study explores, for the first time, the use of porous chemical compounds, called crown ethers, as mechanisms to create pores in electrolytes that can hold and transport lithium ions. One of the challenges for successful implementation is the size of the pores in the crown ether, said Praveen K. Thallapally, the PNNL materials scientist who led this work.
“The pore size needs to be just right,” said Thallapally, “and that would mean large enough to hold lithium, but also small enough to prevent the lithium from slipping back into the surrounding liquid. That would reduce efficiency.”
The concept of porous electrolytes deserves more study, Murugesan said.
“What we found and what we show in this study has great potential,” he said. “Properly managed, porous compounds can provide a pathway to a more efficient next-generation battery.”
Study authors in addition to Murugesan and Thallapally include Sun Hae Ra Shin, Hyung-Seok Lim, Kee Sung Han, Alexander J. Robinson, Aaron Hollas, Bhuvaneswari M. Sivakumar, Samantha I. Johnson, Jaehun Chun, and Wei Wang.
Published: February 21, 2023