Isolation and Characterization of a Rationally Designed Copper Hydride Monomer
Isolating a molecular copper hydride monomer for the first time
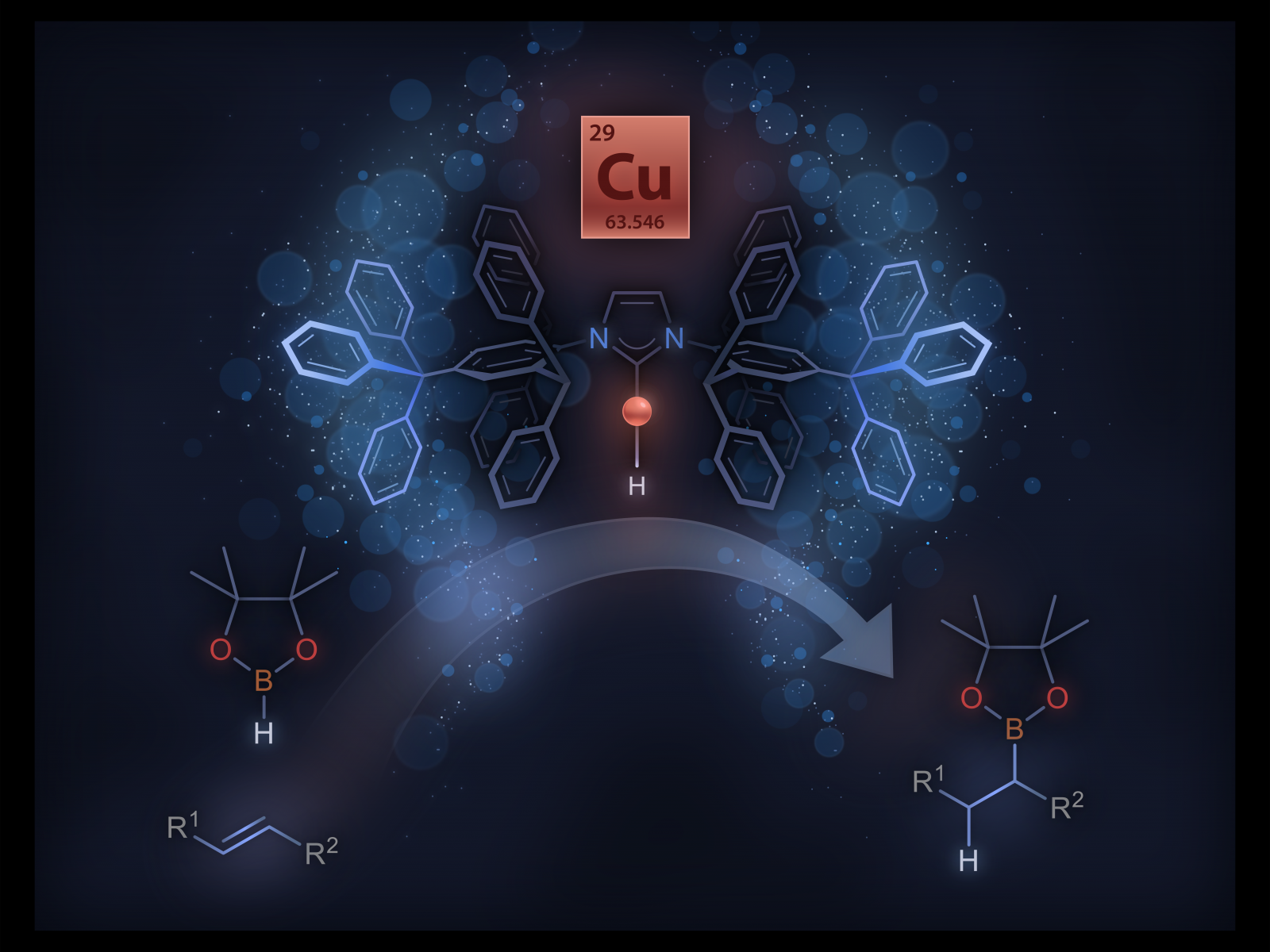
Newly isolated monomeric copper hydride complex can catalyze the hydroboration of unactivated internal alkenes.
(Image by David Ryan | Pacific Northwest National Laboratory)
The Science
Many catalytic reactions require the addition of hydrogen to a molecule. Molecular copper hydrides are particularly useful in catalysis as a range of unsaturated molecules readily insert into the copper-hydrogen bond. Scientists have tried to isolate a molecular copper hydride (Cu-H) monomer for over four decades. Guided by mechanistic studies, researchers designed an N-heterocyclic carbene (NHC) ligand, IPr*CPh3, that enabled the isolation of an elusive two-coordinate (IPr*CPh3)CuH monomer. They were able to characterize the monomer and study its reactivity with other molecules. The monomer can also insert into generally unreactive carbon-carbon double bonds, including unactivated internal alkenes.
The Impact
Copper is a versatile and Earth-abundant metal, with an important role in catalysis. Better understanding how intermediates like copper hydrides form and behave is important for designing next generation catalysts. In addition, this study used the often-overlooked approach of modifying remote steric properties to synthesize NHC ligands capable of stabilizing monomeric Cu-H. This facilitated the first isolation of a Cu-H monomer, which demonstrated previously inaccessible stoichiometric and catalytic reactivity toward challenging unactivated internal alkenes.
Summary
Through rational design of a new NHC ligand, IPr*CPh3, researchers isolated a highly reactive and long sought-after monomeric Cu-H complex. Previous systematic structure-activity studies guided the incorporation of a bulky –CPh3 group on the periphery of the NHC ligand, seven bonds from the copper. This feature was critical to the stability and isolation of the monomeric Cu-H complex. The role of remote steric properties in controlling the structure and reactivity of metal complexes is often overlooked in ligand design and catalysis. Access to a clean monomeric Cu-H complex enabled quantitative kinetic studies on the insertion of unactivated internal alkenes, including a less reactive tri-substituted internal alkene. The team identified the important role of the relative concentration of monomeric Cu-H on increasing insertion rate. This work also demonstrated that the monomeric Cu-H complex catalyzes the hydroboration of unactivated internal alkenes, a reaction generally confined to platinum group metals. Systematic investigations are underway to uncover new chemistries of monomeric Cu-H complexes.
The results of this study show the impact of the synergy and close collaboration in the Institute for Integrated Catalysis (IIC; https://iic.pnnl.gov/) between synthetic chemists, kineticists, and X-ray crystallographers to address the longstanding proposal of a fleeting monomeric Cu-H intermediate in molecular Cu-H catalysis.
PNNL Contact
Morris Bullock, Pacific Northwest National Laboratory, morris.bullock@pnnl.gov
Ba Tran, Pacific Northwest National Laboratory, ba.tran@pnnl.gov
Funding
This work was supported by the Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences.
Published: August 30, 2022
Carroll, T. G.; Ryan, D. E.; Erickson, J. D.; Bullock, R. M.; Tran, B. L. 2022. "Isolation of a Cu–H Monomer Enabled by Remote Steric Substitution of a N-Heterocyclic Carbene Ligand: Stoichiometric Insertion and Catalytic Hydroboration of Internal Alkenes." J. Am. Chem. Soc., 114, 13865. DOI: 10.1021/jacs.2c05376.