Heavy Metal Complexation with Ionic Liquids Enables Efficient Electrochemical Separation from Aqueous Solution
The formation of highly reducible lead/ionic liquid complexes opens new opportunities to efficiently separate heavy toxic metals
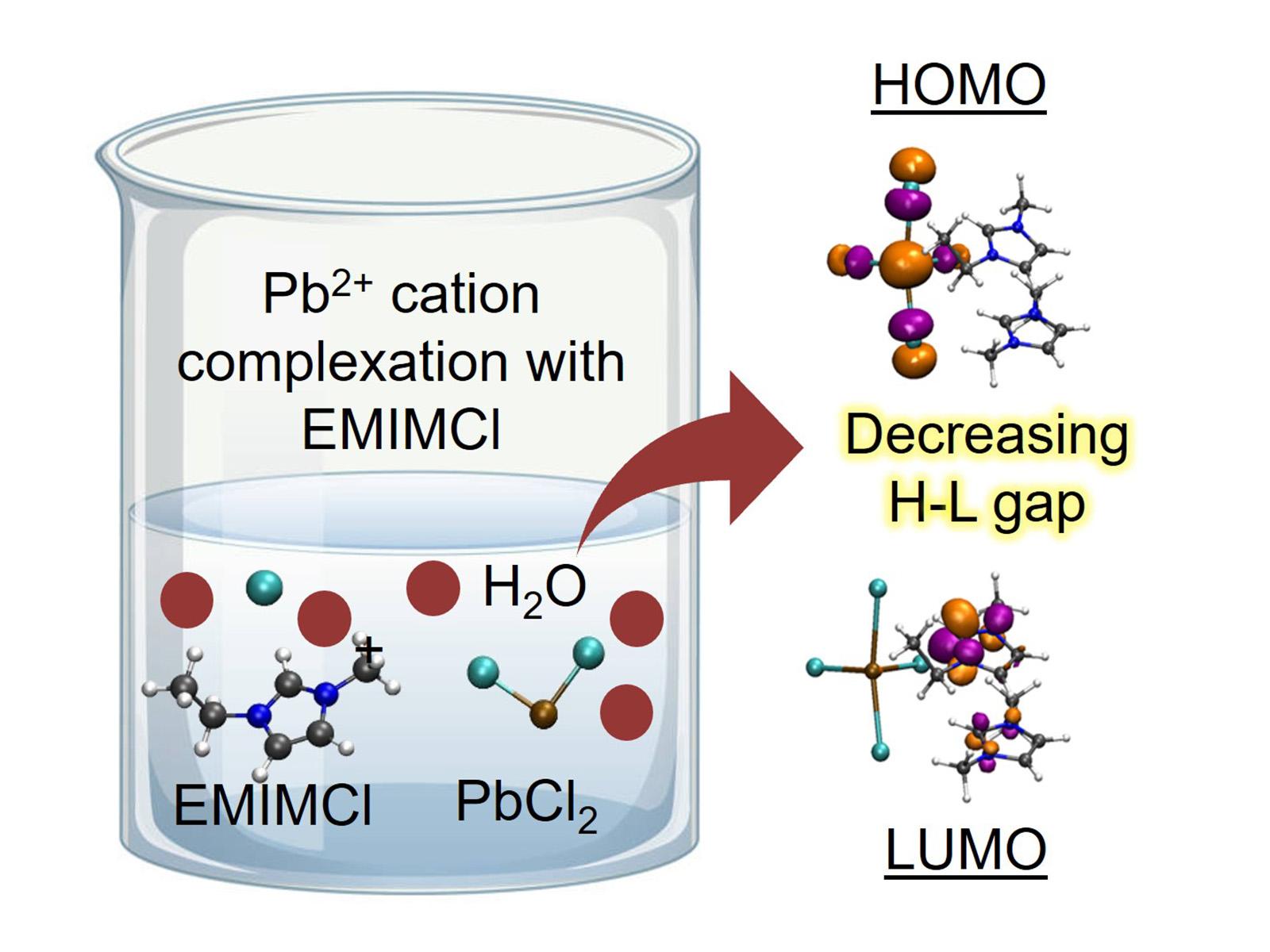
The complexation of metals with ionic liquids reduces the energy gap between the highest occupied and lowest unoccupied molecular orbitals, forming more reducible structures for energy-efficient electrochemical separations.
(Image by Venky Prabhakaran | Pacific Northwest National Laboratory)
The Science
Understanding the behavior of and interactions between ions and molecules in solution is essential for effective separations. Researchers studied how imidazolium-based ionic liquids (ILs) combine with lead cations in both the gas phase and solution. They found that the lead and ILs formed complexes differently in solution than in the gas phase. The presence of hydrogen bonding from water affected the electrostatics of the bonds between the lead and chloride ions and the IL, resulting in different more reducible and separatable complexes. This work provides insight into how solvents change the fundamental interactions between molecules and control the final complexes that form.
The Impact
The practical decontamination of industrial wastewater and recovery of critical materials depend on the ability to rationally design energy-efficient separation processes. By developing a better understanding of how greener molecules, such as ILs, interact with elements of interest, researchers may devise more effective separation schemes. This understanding is particularly important for separations based on electric fields, where tuning the properties of complexes has a substantial effect on the efficiency and selectivity of the separation process.
Summary
ILs are exciting for applications in separations and other fields due to their ability to trap targeted molecules as well as their highly tunable chemical and physical properties. Pristine ILs have been explored to selectively form complexes with different heavy metal ions and rare-earth elements, but their application in practical separations remains limited as most feed streams are aqueous. Researchers explored the possibility of using ionic liquids as co-solvents or diluents for aqueous solutions. They studied the electronic and geometric properties of complexes formed between imidazolium-based ILs and lead cations in both gas- and aqueous-phase environments. They found that redistribution of the hydrogen bonding between the IL cations and anions in the presence of solvating water molecules leads to increased electrostatic screening of bonds between lead and chloride. In water, this screening results in the formation of complexes different from those observed in the gas phase, providing important insight into the role of solvent in controlling molecular interactions and speciation.
Contact
Venky Prabhakaran, Pacific Northwest National Laboratory, venky@pnnl.gov
Grant Johnson, Pacific Northwest National Laboratory, grant.johnson@pnnl.gov
Manh-Thuong Nguyen, Pacific Northwest National Laboratory, manhthuong.nguyen@pnnl.gov
Funding
This work was supported by the Department of Energy (DOE), Office of Science, Basic Energy Sciences program, Chemical Sciences, Geosciences, and Biosciences Division, FWP 81462 (Harnessing Confinement Effects, Stimuli, and Reactive Intermediates in Separations). The negative ion photoelectron spectroscopy work was supported by DOE, Office of Science, Basic Energy Sciences program, Division of Chemical Sciences, Geosciences, and Biosciences, Condensed Phase and Interfacial Molecular Science program, FWP 16248. Pacific Northwest National Laboratory (PNNL) is a multiprogram national laboratory operated by Battelle for the DOE under Contract DE-AC05-76RL01830. Computer resources were provided by Research Computing at PNNL, and the National Energy Research Scientific Computing Center (NERSC), a DOE Office of Science user facility operated under Contract No. DE-AC02-05CH11231.
Published: April 26, 2024
Tan, S.; Zhang, D.; Chen, Y.; Helfrecht, B. A.; Baxter, E. T.; Cao, W.; Wang, X.-B.; Nguyen, M.-T.; Johnson, G. E.; Prabhakaran, V. “Complexation of heavy metal cations with imidazolium ionic liquids lowers their reduction energy: implications for electrochemical separations,” Green Chemistry 26, 1566-1576 (2024). [DOI: 10.1039/D3GC03713D].