Artificial Intelligence Drives Design, Discovery, and Testing of Targeted Therapeutics against SARS-CoV-2
Data-driven autonomous technology to rapidly design and deliver antiviral interventions targeting SARS-CoV-2
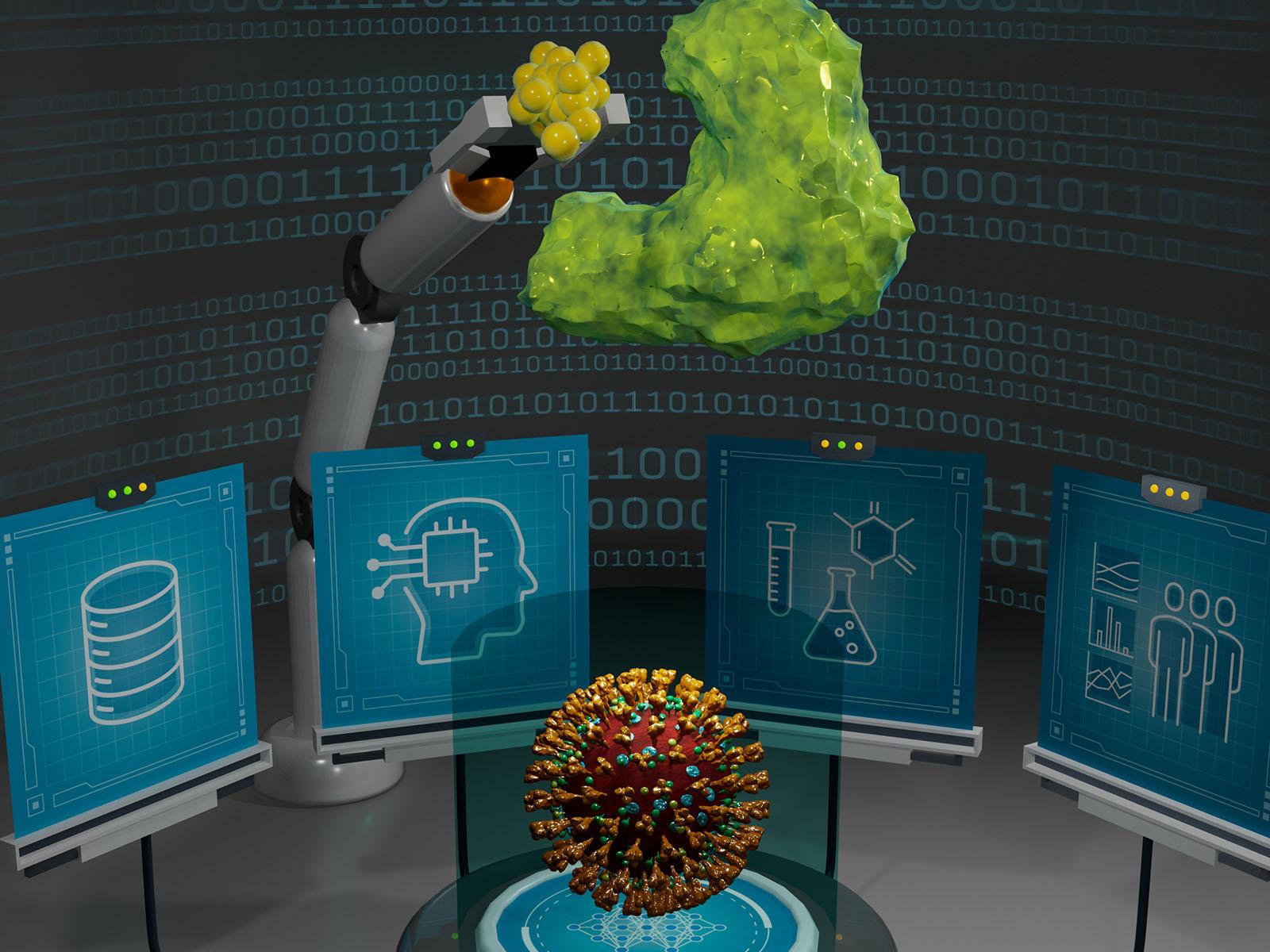
Illustration representing a closed-loop deep learning-assisted automated workflow for designing electrophilic warhead-based covalent inhibitors targeting SARS-CoV-2 main protease.
(Illustration by Nathan Johnson | Pacific Northwest National Laboratory)
The Science
The SARS-Co-V-2 virus responsible for COVID-19 can be treated by both vaccines and antivirals. Vaccines provide immunity, but antivirals treat infection. Given the rapid evolution of new variants, it was necessary to streamline the discovery, production, and testing of new antivirals. Many antiviral therapeutics target and interfere with a key coronavirus protein Mpro, otherwise known as main protease. Mpro is responsible for copying and replicating the virus. Therapeutics bind with Mpro and shut the virus down.
Interactions between “warheads” that deliver therapeutics and main protease utilize a specific type of chemical bond. These interactions can broadly be divided into two types—noncovalent and covalent. To date, research has focused primarily on noncovalent interactions. This study presents a closed-loop system that rapidly designs, and tests, therapeutic warhead delivery based on covalent interactions with main protease.
The system begins with an artificial intelligence (AI) based deep learning (DL) model to design warhead based novel drug candidates, producing those designs on a three-dimensional (3-D) scaffold, and then testing the delivery of the therapeutics against Mpro using high throughput virtual screening, functional, cell-based assays, native mass spectrometry, and x-ray crystallography.
The Impact
Potentially successful candidates were assessed using experimental methods, closing the loop of design, delivery, and validation. Using the closed-loop workflow system leverages all possible warheads. Through this process, promising candidates in the library were screened based on their binding affinity and interactions, which indicated their potential effectiveness as protease inhibitors.
Several potential candidates were identified and further tested experimentally using native mass spectrometry and fluorescence resonance energy transfer (FRET)-based screening assays, both to demonstrate the viability of our approach and because the experimental data are key to the successive iterations of optimizations. Together, our strategy provides a scalable platform for the rapid discovery of viable covalent lead candidates not only targeting SARS-CoV-2 but also for other emerging threats.
Summary
Direct-acting antivirals for the treatment of COVID-19 pandemic caused by the SARS-CoV-2 virus is needed to complement vaccination efforts. Given the ongoing emergence of new variants, fast workflows for antiviral discovery remains critical to our ability to address the pandemic’s evolution in a timely manner. While several such pipelines have been introduced to discover candidates with potential for noncovalent interactions with the main protease (Mpro), the research team at Pacific Northwest National Laboratory (PNNL) developed a closed-loop AI pipeline to design covalent candidates.
This work introduced a DL-assisted automated computational workflow to introduce linkers and an electrophilic “warhead” and incorporated cutting-edge experimental techniques for validation. Through this process, promising candidates in the library were screened and several potential hits were identified and tested experimentally. This multidisciplinary approach used Department of Energy’s (DOE’s) capabilities and user facilities, to complement public and private sector resources for accelerating discovery of medical therapeutics targeting SARS-COV-2.
PNNL Contact
Neeraj Kumar, Pacific Northwest National Laboratory, Neeraj.Kumar@pnnl.gov
Funding
This research was supported by the DOE Office of Science through the National Virtual Biotechnology Laboratory, a consortium of DOE national laboratories focused on response to COVID-19, with funding provided by the Coronavirus CARES Act. Computing resources was supported by the intramural program at the Environmental Molecular Sciences Laboratory (EMSL), a DOE Office of Science user facility at PNNL. Additional resources were supported by Laboratory Directed Research and Development program at the PNNL.
Published: February 21, 2023
Joshi R.P., K.J. Schultz, J.W. Wilson, A. Kruel, R.A. Varikoti, C.J. Kombala, D.W. Kneller, S. Galanie, G. Phillips, Q. Zhang, L. Coates, J. Parvathareddy, S. Surendranathan, Y. Kong, A. Clyde, A. Ramanathan, C.B. Jonsson, K.R. Brandvold, M. Zhou, M.S. Head, A. Kovalevsky, and N. Kumar. “AI-Accelerated Design of Targeted Covalent Inhibitors for SARS-CoV-2.” Journal of Chemical Information and Modeling (2023). [DOI: https://doi.org/10.1021/acs.jcim.2c01377]