Molecular Catalysis
What is molecular catalysis?
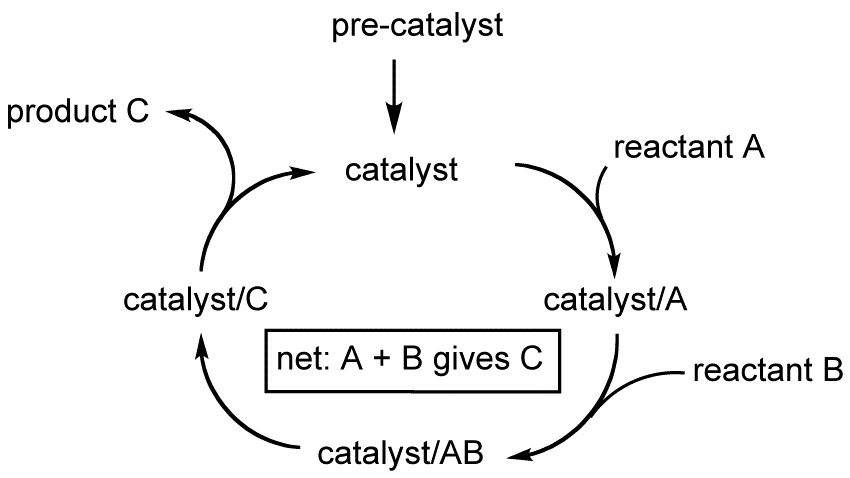
Catalysis is the process of speeding up a chemical reaction by adding a compound—a catalyst—that is the same at the start and end of the reaction. Catalysts allow scientists to perform reactions that would be too slow to be useful without a catalyst present. These reactions range from those needed to convert small organic, or carbon-based, molecules into more valuable products or to make pharmaceuticals. Molecular catalysis, as used here, specifically refers to catalysis where all components of the reaction are dissolved in the same liquid phase.
Molecular catalysis plays a large role in numerous industries where having precise control over chemical reactivity is key. Molecular catalysts possess extensive tunability in size, composition, and reactivity. The ease of adjusting catalyst properties, or the tunability, for molecular catalysts represents an advantage over other types of catalysts (e.g., solid-state catalysts) and allows comparative studies that isolate the effects of specific structural or electronic changes to the catalyst on reactivity. Many molecular catalysts are organometallic, containing a metal bonded to organic molecules called ligands, which can be swapped out or chemically modified to enhance reaction rates.
Many analytical techniques exist for studying small-molecule systems in detail, making them ideal models for developing fundamental scientific understanding of catalytic processes. Using knowledge of how these catalysts work enables researchers to tune them to effectively produce a wider range of chemicals more easily.
Molecular catalysis history
Catalytic activity in chemical transformations—the conversion of one chemical to another via chemical reactions—has been observed and studied since ancient times, However, the study of molecular catalysis only began to take shape around the 18th century. Research on molecular catalysts progressed during the early and mid-20th century, especially after the development of analytical chemical techniques like mass spectrometry and nuclear magnetic resonance spectroscopy. The use of increasingly sophisticated ligands coupled to these powerful analytical techniques finally produced detailed molecular-level insight. These analytical tools have enabled researchers to develop a mechanistic understanding of different reactions, with computational tools becoming increasingly important for gaining further information about complex systems.
The importance of molecular catalysis
Molecular catalysis is a key component of the overall field of catalysis and here specifically refers to solution-state homogeneous catalysis where all components are in the solution phase. Molecular catalysts may be composed of a wide range of different atoms, but many involve a metal bonded to one or more carbon-containing molecules referred to as ligands. In a molecular catalyst, the metal tends to act as the active site, to which the reactant binds before undergoing conversion to the desired product.
Ligands play an important role in determining overall catalyst properties and can control the solubility, reactivity, and stability of the whole complex. Developing new ligands is an important part of molecular catalysis research, where small changes to ligand structure can have substantial impact on the catalyst’s efficiency.
Many molecular catalysts have a broad role in chemical transformations and can be studied in detail. This allows scientists to develop an in-depth understanding of chemical reaction processes. Chemical transformations often follow a multi-step process of separate and sometimes coupled reactions that result in an overall change. Previously known reactions can be used to build up a stepwise cycle of how the catalyst facilitates a specific chemical transformation.

Molecular catalysis plays an important role in the synthesis of specialty chemicals, particularly those with complex molecular structures. Many pharmaceutically relevant chemicals include numerous reactive groups and require highly selective catalysts to synthesize. Molecular catalysts can specifically target segments of a molecule or a single type of functional group. The targeting ability derives from the extensive tuning parameters of molecular catalysts, which generally have higher specificity than other catalyst classes.
A major example of the industrial use of molecular catalysis is the production of acetic acid, which is used to make polymers in paint and adhesives and is the primary flavor component of vinegar. In this process, methanol and carbon monoxide combine to generate the desired acetic acid. Commercial production involves a ruthenium or iridium based catalyst in solution and demonstrates the active role molecular catalysis plays in the chemical industry.
The benefits of molecular catalysis
Using a molecular catalyst has significant benefits because these catalysts can offer high levels of tunability and selectivity. Selectivity is particularly important for molecules that are either complex or have multiple sites for reactions. The ability to target a specific portion of a molecule for a reaction facilitates the synthesis of more complex molecules that might have several different reactive groups.
A major strength of molecular catalysts is their highly tunable nature, with various properties that can be precisely controlled. Many metal-centered catalysts have several ligands, all of which can be modified to alter the reactivity. These ligands can be replaced with a completely different molecule or slightly modified with a similar base structure. The metal center is also changeable, and the same set of ligands can be applied to different metals or the same metal in different oxidation states. This allows researchers to compare reactivity and to observe how changes to a wide range of chemical and physical parameters affect catalysis.
An additional benefit of working with molecular catalysts is their amenability to a suite of analytical techniques that allow detailed measurements of the catalytic process. These small-molecule-targeted tools help researchers identify reactions and intermediates, as well as quantify the success of the catalyst. Probing the reaction steps in detail offers a deeper understanding of how the catalysts work and provides insight into modifying catalysts to create more efficient reactions.
Challenges of molecular catalysis
Molecular catalysis is necessarily limited in scope to fully molecular systems, where all components are molecules. These molecular systems present challenges when compared to solid-phase catalysts. One salient difficulty can be isolating the catalyst from the rest of the reaction mixture when everything is dissolved in the same solution. Separating the products and the catalyst often requires costly and labor-intensive purification.
Separating the catalyst from the reaction mixture is particularly important when the catalyst is expensive to produce. Many metal-containing molecular catalysts use metals like ruthenium, iridium, and platinum, all of which are rare and costly. Highly designed catalysts can also require significant time and expertise to produce, making their generation challenging.
Molecular catalysts also face limitations due to their stability. Highly stable solid-phase catalysts commonly operate at high temperatures, but molecular catalysts often degrade at temperatures as low as 100 °C. Significant research efforts have led to more stable molecular catalysts that can operate near 200 °C, but that level of thermal stability is restricted to only a subset of catalysts. Deactivation of catalysts can occur through reactions with chemicals unintentionally present in the mixture, ranging from oxygen to side products of the reaction. This deactivation, often referred to as poisoning, is an issue for all classes of catalysts and requires careful study to avoid.
Recent work in molecular catalysis
Much of the foundational work in molecular catalysis used rare metals that have limited abundance on Earth. While these elements possess properties that make them practical to use as catalyst centers, recent efforts have begun to push the field toward using more Earth-abundant metals. This push has led to increasing studies of metals such as nickel, copper, and iron. In addition to investigating new metal centers, different ligands are necessary to exploit the full potential of these molecules as efficient catalysts. This has led to research centered on increasingly complex ligands. These precisely designed molecules often have extremely long syntheses and are highly tuned to display desired properties. This tuning can be from a steric perspective, because the size of the ligands influences how substrates can bind to the catalyst, or it can be from an electronic perspective, because ligands can add or remove electron density at the metal center in an organometallic complex. Ligands are often more than bystanders to the catalytically active metal center and can cooperate to influence catalysis. Referred to as metal–ligand cooperation, this is an increasingly active field of study.
Some ligands are designed to bind two metals rather than one. The resulting bimetallic species harness the properties of multiple metals, which can be the same or different, and can catalyze more complex reactions. For example, a bimetallic catalyst can have two active sites that bond to different substrates and hold them in close proximity to each other. Alternatively, the two metals can associate with different portions of the same substrate to increase the overall reactivity of the molecule and activate a particularly challenging target.
Molecular catalysis at PNNL
PNNL has a rich history of work within molecular catalysis and has been a leader in this area for many years. PNNL’s research has generally focused on catalytic hydrogenations, adding and removing hydrogen to and from molecules, and electrocatalysis where electricity is used to drive chemical reactions. The efforts at PNNL have focused on many different transformations largely centered around the generation and utilization of fuels. They specifically include the production and oxidation of hydrogen, reduction of CO2, oxidation of formate, oxidation of alcohols, reduction of nitrogen, oxidation of ammonia, and hydrodesulfurization.
Researchers at PNNL have been active collaborators and hosts to visiting scientists due to their expertise and instrumental capabilities, including high-pressure operando nuclear magnetic resonance (NMR) spectroscopy and high-pressure electrochemical capabilities. Recent visitors have included students through the U.S. Department of Energy’s Office of Science Graduate Student Research Program and university faculty through the Visiting Faculty Program. Molecular catalysis researchers, including Morris Bullock, John Linehan, Wendy Shaw, and Eric Wiedner, have been actively engaged as hosts and collaborators and have contributed to large, multi-institutional projects.